The Drug Supply Chain Security Act, or DSCSA, establishes national standards for securing the United States prescription drug channel from the pharmaceutical manufacturer all the way to the dispenser.
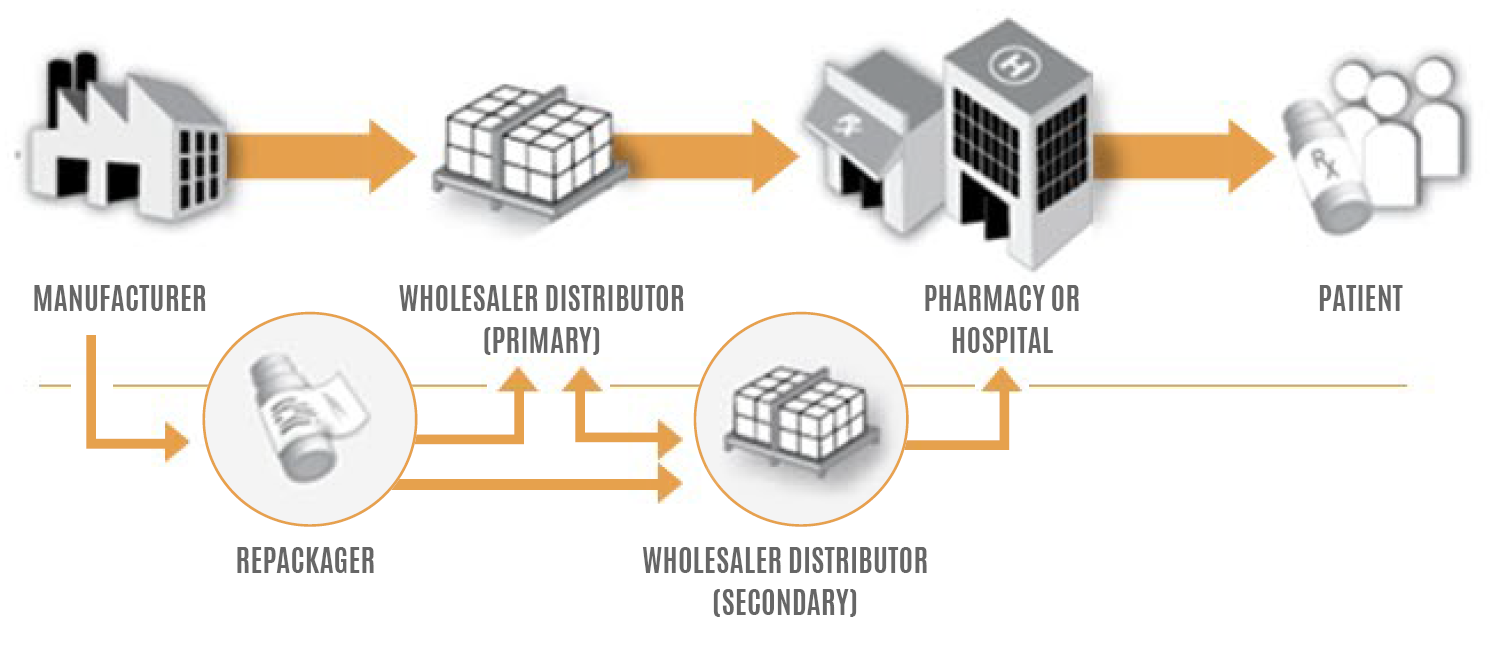
The goals of legislation are to allow for greater trust and transparency while standardizing data exchange and providing a way to recognize suspect and illegitimate products through serialization.
However, it will also bring new rules and regulations to drug companies, creating an increased opportunity for potential mistakes and pitfalls. Mistakes that can cost you time and money by preventing the distribution of products and incurring fines/penalties (even jail time).
The DSCSA has several provisions, allowing the FDA the authority to draft additional requirements and enforcement criteria, which can suddenly put your drug out of compliance. We will remain vigilant, watching for any changes and adjusting plans to make sure you remain DSCSA compliant.
How We Can Help
To remain compliant with the DSCSA, serialization is key; however, a number of new Standard Operating Procedures (SOPs) must be established, new data connections must be formed, and additional barcoding and labeling requirements must be integrated into your packaging plans.
Two Labs can leverage industry experience and knowledge to keep you in good standing through this emerging and seemingly ever-changing system:
Provide neutral, 3rd party education on the DSCSA requirements so your organization is fully aware of regulatory obligations
Attend industry conferences and participate in DSCSA working groups to ensure you are on the forefront of industry best practices
Stay up to date on new FDA guidance documents
Advise your organization through the serialization data provider selection process
Assist in creation of required master data elements for serialization
Project Management support with the integration of serialization system(s) and your packaging and trading partners
Provide guidance and assistance to ensure SOPs are in alignment with DSCSA requirements and your internal quality procedures
Review/scan labels to ensure they meet HDA & GS1 guidelines (which downstream trading partners require)
Advise your packager when deficiencies are identified
At Two Labs, we’re excited to see what new capabilities the DSCSA brings to the pharmaceutical industry, but we also realize that it will put a lot of stress on drug manufacturers. We’re here to help lighten the load, making sure your transition to DSCSA compliance is as easy as possible.
If you have any questions about Two Labs’ capabilities and how we can help with ensuring your DSCSA compliance, contact us for a consultation.
Contact Us